
Explanation: The ideal environmental conditions for a reaction, such as temperature, pressure, catalysts, and solvent. A common threshold to describe something as insoluble is less than 0.1 g per 100 mL of solvent. Further information about equation 2Ag + 2H 2 SO 4 2H 2 O + SO 2 + Ag 2 SO 4 What is reaction condition of Ag (silver) reacts with H2SO4 (sulfuric acid) No information found for this chemical equation.

Use E(cell) to find Qc for these concentrations, assuming that the silver sulfate precipitate is in equilibrium with its ions already.
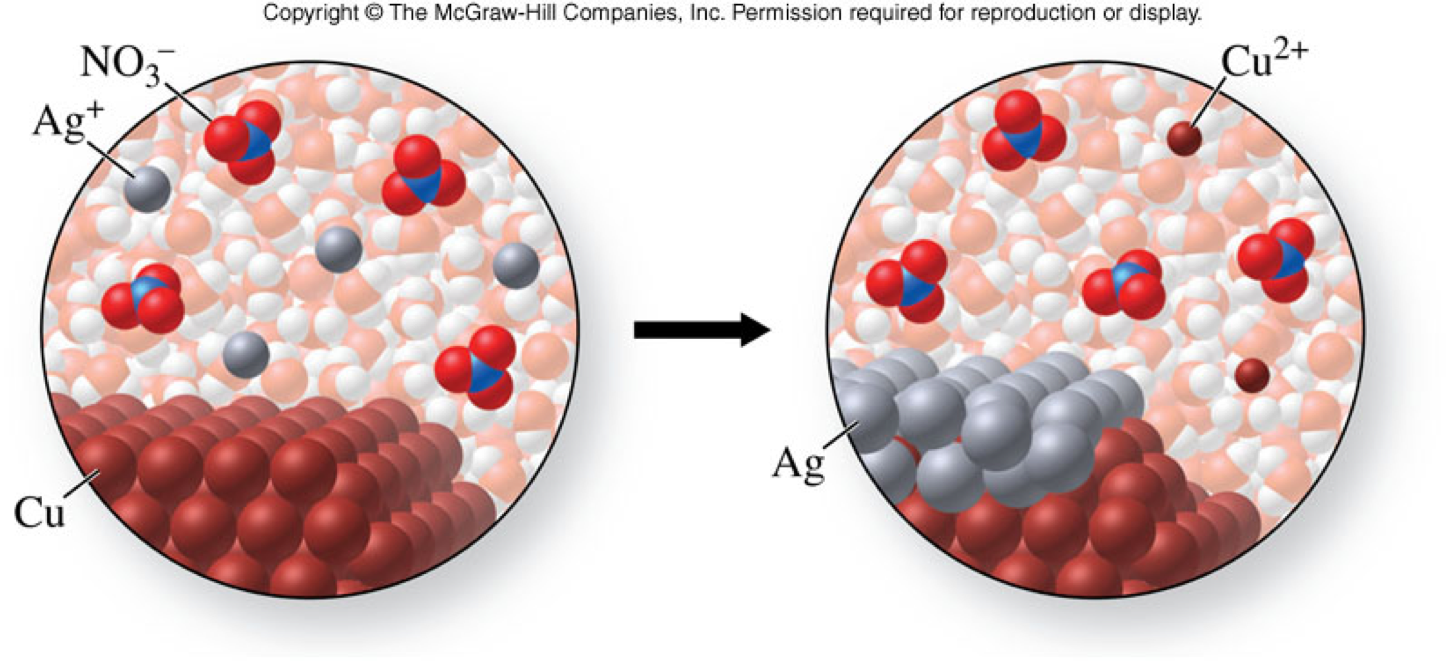
Utiliza la calculadora de abajo para equilibrar las ecuaciones químicas y determinar el tipo de reacción (Instrucciones). It looks like the point of this is to: Find E(cell) using reference values you should be given. Calcula la estequiometría de las reacciones químicas. The term insoluble is often applied to poorly or very poorly soluble compounds. 2NH 3 (g) + H 2SO 4 (aq) (NH 4)2SO 4 (aq) 0.00568 grams NH 3 grams (NH 4)2SO 4 2.21 x 10-2 g (NH 4)2SO 4 2NO (g) + O 2 (g) 2NO 2 (g) 6.50 moles O 2 moles NO 2 13. Cuprifera Cuprifero Cuprífera Cuprífero Cu Masa molar Cu Número de oxidación. The solubility of a substance is an entirely different property from the rate of solution, which is how fast it dissolves. Electronic configuration of Ag+ is 4d10 5s0, Hence it contains all paired electrons with.

The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution and begins to precipitate the excess amount of solute. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and the pH of the solution. Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent. Create an equation for each element (Mg, Ag, S, O) where each term represents the number of atoms of the element in each reactant or product. Question: Is Ag2SO4 ( Silver sulfate ) Soluble or Insoluble in water ?Īnswer: Ag2SO4 ( Silver sulfate ) is Insoluble in water


 0 kommentar(er)
0 kommentar(er)
